Presentation Information
[R1-P-16]Titantaramellite from the Tanohata mine, Iwate Prefecture, NE Japan
*Shunsuke Ohsumi1, Mariko Nagashima2, Hiroki Oka3 (1. Non, 2. Yamaguchi Univ. Sci., 3. OYO Corp.)
Keywords:
titantaramellite,nagashimalite,Tanohata mine
The Tanohata mine in the Tanohata village, Iwate Prefecture, northeastern Japan, is a well-known manganese mine as the type locality of several minerals. Additionally, V-rich silicate minerals, including nagashimalite and momoiite, have been reported (Matsubara et al., 2010). In this study, we report the first occurrence of titantaramellite in Japan.
Titantaramellite, Ti4+-analogue of nagashimalite, was reported from the West Coast of the USA in the process of re-examining taramellite. It is associated with mainly Ba-silicate minerals in the contact zone between granitic bodies and metamorhosed sedimentary rock.
Titantaramellite from the Tanohata mine was found in siliceous manganese ores as yellowish dark green flat crystals, up to 1 mm in diameter, associated with rhodonite (sensu lato), yoshimuraite, pyrophanite, Sr-bearing apatite, alkali-amphiboles, and small amounts of metallic minerals.
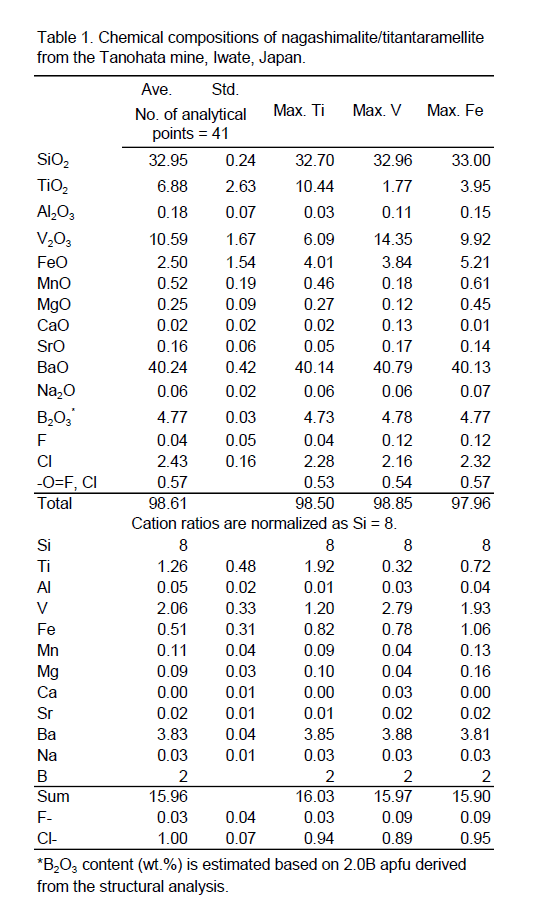
The results of quantitative analysis by EPMA are shown in Table 1. The contents of Ti, V, and Fe vary as follows: TiO2 1.77–10.44, V2O3 6.09–10.44, FeO 0.77–5.21 wt%. Chemical distributions of V, Ti, and Fe randomly change in a single crystal. The average of the 41 analyzed points indicates V3+-dominant, nagashimalite, whereas seven of them are Ti4+-dominant, titantaramellite.
Crystallographic analysis of titantaramellite from the Tanohata mine shows that boron (B) fully occupies tetrahedral B site, while Alfors and Pabst (1984) indicated the presence of partial vacancy in the B site. Since the position of H was not determined, it is assumed that OH is not present, or present in very small amounts, replaced by O2-.
Assuming that all Fe are Fe3+, the charge on the M-site is 3.27, and in this case the total charge on the cation (59.08) exceeds the total charge on the anion (-59) in the absence of OH. It is indicated that in part of the Fe is divalent, or all of the Fe is trivalent, and a small amount of OH existing.
Titantaramellite, Ti4+-analogue of nagashimalite, was reported from the West Coast of the USA in the process of re-examining taramellite. It is associated with mainly Ba-silicate minerals in the contact zone between granitic bodies and metamorhosed sedimentary rock.
Titantaramellite from the Tanohata mine was found in siliceous manganese ores as yellowish dark green flat crystals, up to 1 mm in diameter, associated with rhodonite (sensu lato), yoshimuraite, pyrophanite, Sr-bearing apatite, alkali-amphiboles, and small amounts of metallic minerals.
The results of quantitative analysis by EPMA are shown in Table 1. The contents of Ti, V, and Fe vary as follows: TiO2 1.77–10.44, V2O3 6.09–10.44, FeO 0.77–5.21 wt%. Chemical distributions of V, Ti, and Fe randomly change in a single crystal. The average of the 41 analyzed points indicates V3+-dominant, nagashimalite, whereas seven of them are Ti4+-dominant, titantaramellite.
Crystallographic analysis of titantaramellite from the Tanohata mine shows that boron (B) fully occupies tetrahedral B site, while Alfors and Pabst (1984) indicated the presence of partial vacancy in the B site. Since the position of H was not determined, it is assumed that OH is not present, or present in very small amounts, replaced by O2-.
Assuming that all Fe are Fe3+, the charge on the M-site is 3.27, and in this case the total charge on the cation (59.08) exceeds the total charge on the anion (-59) in the absence of OH. It is indicated that in part of the Fe is divalent, or all of the Fe is trivalent, and a small amount of OH existing.